Vertex Pharmaceuticals is No. 11 on the list of the World’s 50 Most Innovative Companies of 2024. Explore the full list of companies that are reshaping industries and culture.
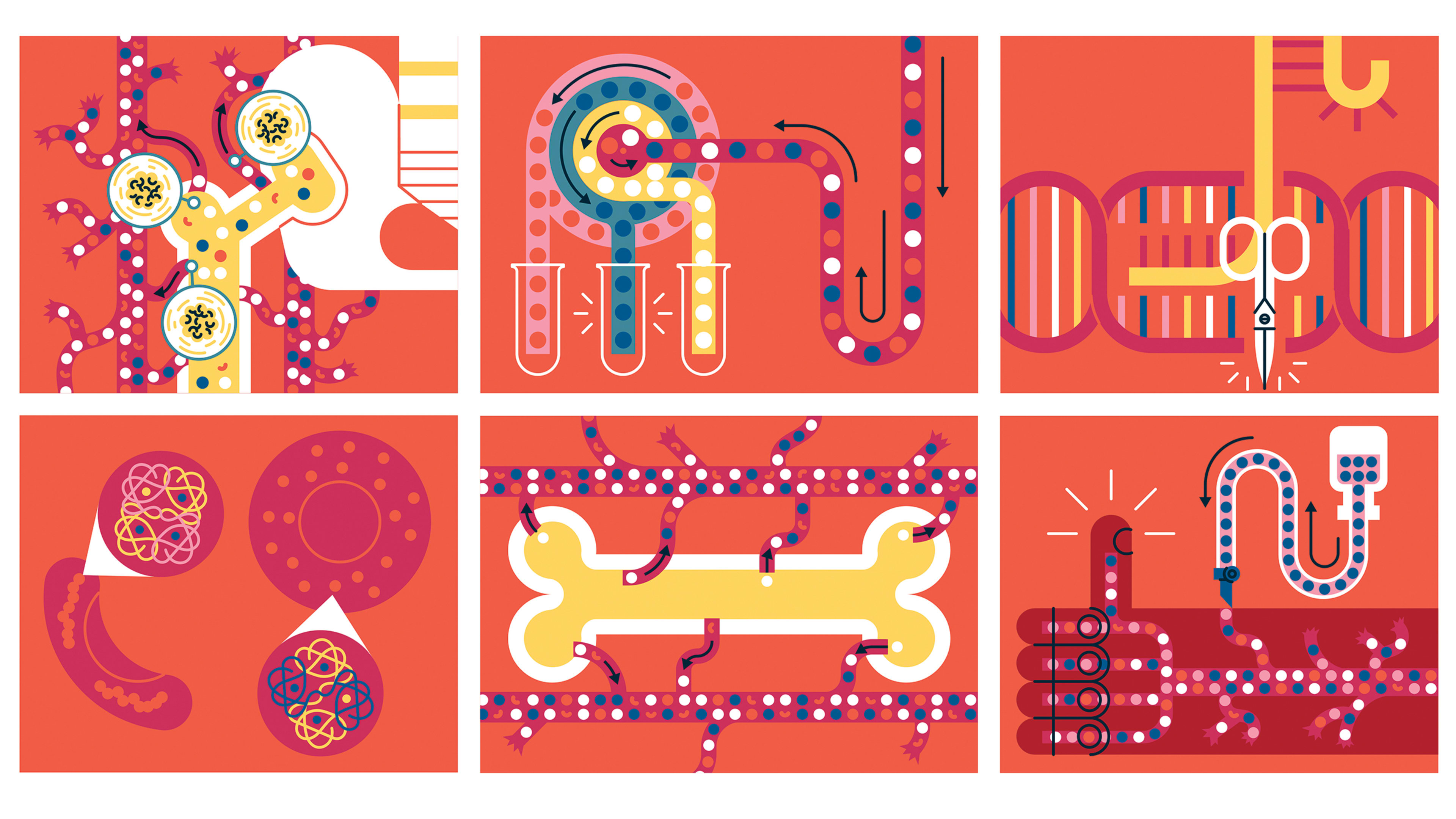
Until late 2023, there was just one cure for sickle cell disease: a bone marrow transplant from a matched donor. The vast majority of patients for whom this wasn’t an option could only hope to use medication and blood transfusions to manage the pain and potential organ damage that results from the illness, which is caused by a mutation that makes red blood cells develop in a flow-limiting crescent shape.
But in December, the Food and Drug Administration (FDA) approved Casgevy, both a “functional cure” for sickle cell and the first-ever therapy to deploy the revolutionary gene-editing tool CRISPR-Cas9. Codeveloped by Vertex Pharmaceuticals and CRISPR Therapeutics for people 12 years and older, Casgevy works by editing a patient’s blood stem cells to turn on the production of oxygen-rich “fetal hemoglobin,” which is normally turned off soon after birth.
The drug marks a breakthrough for the inherited disorder, which affects some 100,000 Americans, most of whom are Black. When a patient’s modified cells are returned to their body via Casgevy, they produce red blood cells with healthy oxygen levels, alleviating sickle cell disease symptoms. Shortly after it was cleared to treat sickle cell, in January 2024, the FDA approved Casgevy as a therapy for beta thalassemia, another inherited blood disorder affecting hemoglobin production.
The approvals are landmarks for the biotech industry, coming just 12 years after the CRISPR-Cas9 system was first described by Jennifer Doudna and Emmanuelle Charpentier, who won a Nobel Prize in Chemistry in 2020 for their work. Casgevy is the first treatment to emerge from Vertex’s strategic collaboration with CRISPR Therapeutics: A joint team worked on R&D and developed the manufacturing process, while in 2021 Vertex took the lead on the program and now manages all aspects of Casgevy worldwide. .
But CRISPR is beside the point, says David Altshuler, Vertex’s chief scientific officer. “In our industry, people often think in terms of technologies,” he says, “and I think that’s why often they stumble.” Altshuler says Vertex identifies “serious diseases with great unmet need, where there’s the combination of a deep insight into the causal biology and a technology that we can either invent or acquire that allows us to solve that one problem.” To that end, in April 2023 Vertex received expanded FDA approval for Trikafta, a three-drug combination treatment for cystic fibrosis, in patients as young as 2. The company’s pipeline also includes a non-opioid pain signal inhibitor which is being studied in both chronic and acute pain—and recently reported positive results in Phase II and III trials, respectively.
“We still spend [more than] 70% of operating expenses on R&D, and that is unique for a company with nearly $10 billion in revenue,” Altshuler says. Among its R&D efforts: looking for a small molecule treatment for sickle cell disease that would be more accessible than Casgevy in the developing world, where gene editing isn’t feasible. Just don’t call it a copycat drug. “We don’t do any ‘me too’ drugs,” Altshuler says. “Everything we do is innovative.”
Explore the full 2024 list of Fast Company’s Most Innovative Companies, 606 organizations that are reshaping industries and culture. We’ve selected the firms making the biggest impact across 58 categories, including advertising, artificial intelligence, design, sustainability, and more.
Recognize your brand’s excellence by applying to this year’s Brands That Matter Awards before the early-rate deadline, May 3.