Illinois-based Abbott Laboratories has just been granted emergency use authorization by the Food and Drug Administration for its “BinaxNOW COVID-19 Ag Card,” a pocket-sized COVID-19 antigen test that’s already been dubbed a “game changer” by the likes of Assistant Secretary for Health Dr. Brett Giroir.
Here’s what you need to know:
How does it work?
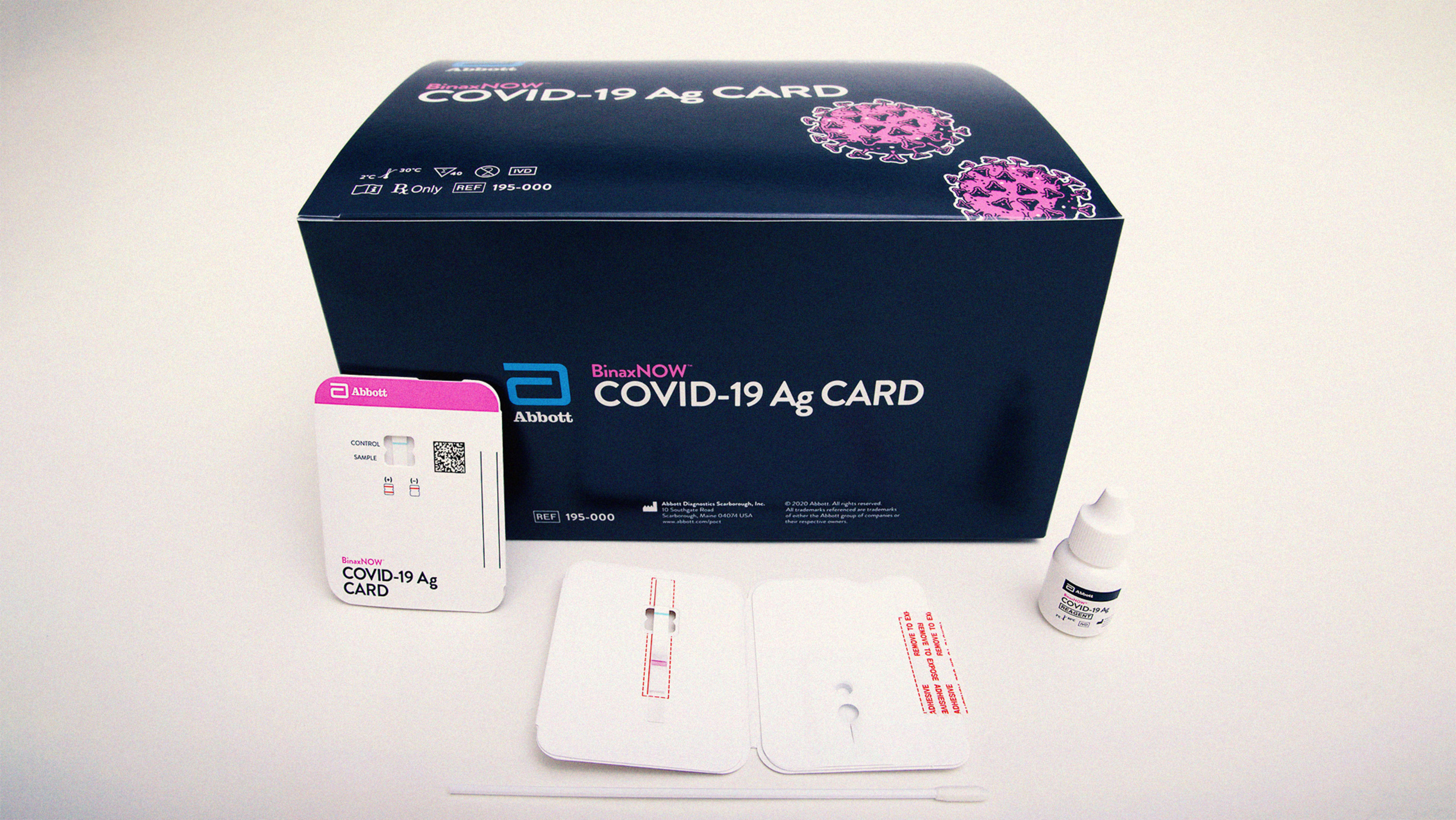
To use BinaxNOW, stick a nasal swab into the device, and a colored line will show up if results are positive for the coronavirus. The swab, by the way, is shorter than the nasopharyngeal Q-tip you may have heard of.
The test is also linked to a free mobile app, which lets users whose results are negative for the coronavirus display a “digital health pass.”
What’s different about it?
BinaxNOW is an antigen test, meaning it detects proteins that are present on the surface of the coronavirus. This differs from the standard polymerase chain reaction test, which detects the genetic sequence of the virus itself. While antigen tests are considered less accurate than PCR tests, they’re much faster—PCR tests typically take days to return results, which has stymied healthcare providers across the country, but BinaxNOW has a turnaround time of just 15 minutes.
Also, BinaxNOW is cheap, costing just $5 compared to $15-$50 for its market competitors.
This matters because public health officials say quick, ubiquitous testing is critical to stopping COVID-19, and would be key to letting citizens safely go back to schools and offices. Abbott’s test can identify patients for containment without the multi-day lag, during which they could infect others, and its low-cost, mass-production capabilities mean those who need tests can get them.
Furthermore, BinaxNOW requires no lab equipment, in contrast to lab-based PCR tests. Thus it could theoretically be deployed anywhere, which could “help democratize testing, making more tests more available to people in more austere settings that don’t have all the trappings found in a medical office,” says former FDA commissioner Scott Gottlieb.
How accurate is it?
BinaxNOW has a correct positive diagnosis rate of 97.1% and a correct negative diagnosis rate of 98.5% when used within the first seven days of symptoms, according to Abbott.
When will I be able to get one?
Abbott said it plans to start shipping tens of millions of test kits in September, and will ramp up to 50 million test kits per month in October. The tests will be conducted by medical professionals at their facilities—such as doctors, school nurses, and pharmacists—and will not be sold direct to consumers.
Recognize your brand’s excellence by applying to this year’s Brands That Matter Awards before the early-rate deadline, May 3.