In the near-future world of David Cronenberg’s 1999 thriller eXistenZ, much of the world’s population has had a “bioport” installed in their spine, allowing them to directly interface with immersive multiplayer video games. Hardware and other forms of technology have become biological, organic, and squishy. (It’s not a film for squeamish viewers.)
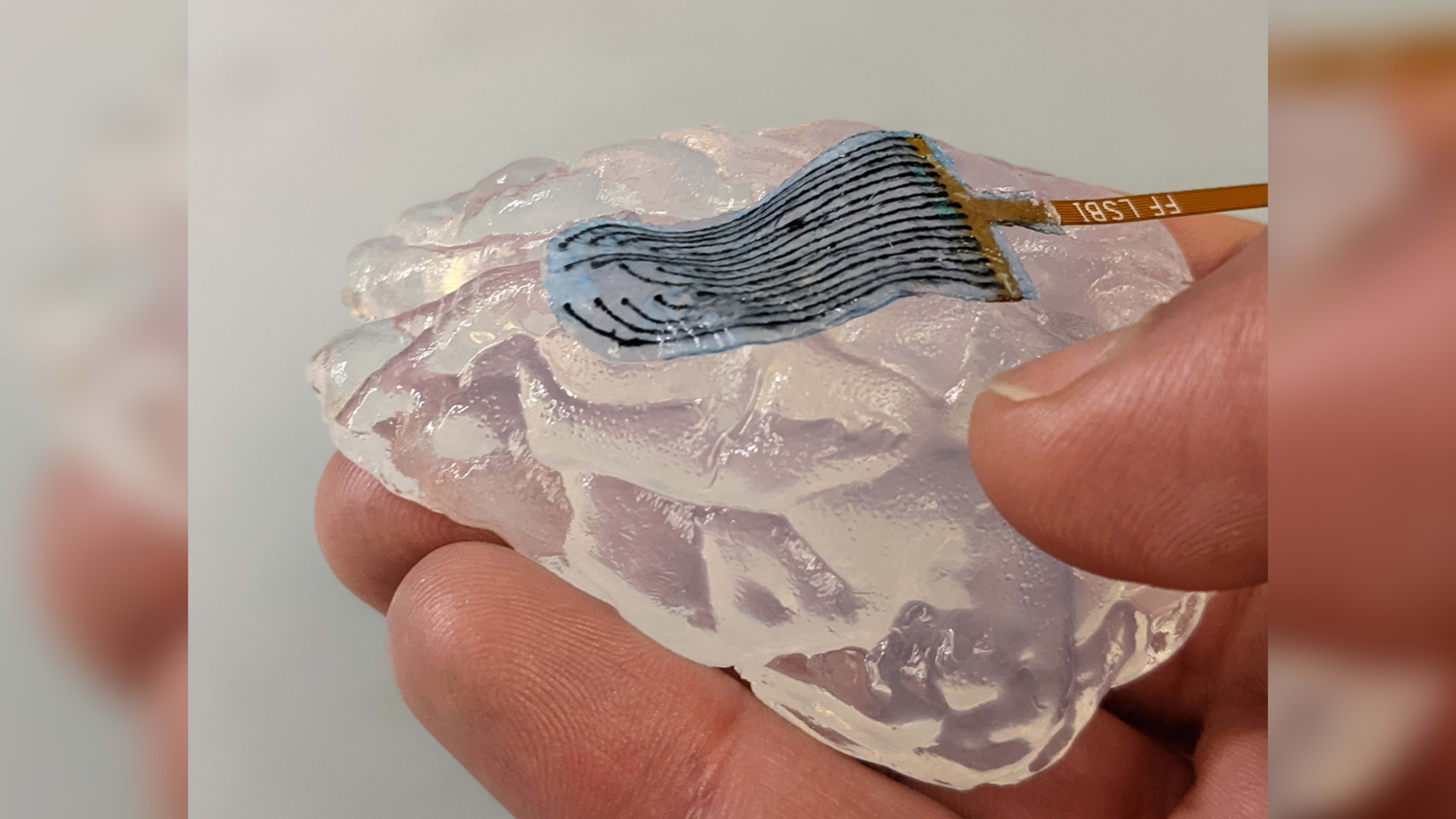
The director’s vision is getting closer to reality. In a recent article in Advanced Healthcare Materials, researchers from the Wyss Institute at Harvard University, Harvard’s John A. Paulson School of Engineering and Applied Sciences, and the Massachusetts Institute of Technology reported on a new type of electrically conductive hydrogel “scaffold” that could eventually be used to create a soft brain-computer interface (or BCI) that translates neural signals from the brain into machine-readable instructions.
Numerous companies—including Blackrock Neurotech, BrainGate, Synchron, and Elon Musk’s Neuralink—have BCI technology in development, with potential applications that include monitoring brain activity in people with neurological disorders, restoring vision in the blind, helping people with paralysis to regain control and feeling in their limbs, and enabling humans to control machines (including planes and weapons) using only their mind.
The technology is still largely experimental—a 2022 report from the U.S. Government Accountability estimated that fewer than 40 people worldwide have implanted BCIs, which are surgically attached directly to the patient’s gray matter. (Neuralink is being investigated for animal rights complaints stemming from reports of hundreds of dead animals involved in testing.) So far, only two companies—Blackrock and Synchron—have received permission from the Food and Drug Administration to test BCI implants in humans.

Nearly all the known devices currently in development rely on tiny electrodes made of metal—not a particularly brain-friendly material. They pose the risk of infection and rejection. And because metal is hard and rigid, it can’t truly conform to the contours of the brain, which makes it hard to maintain a secure, reliable connection with the organ’s slippery surface. “An analogy to this stiffness mismatch is placing a metal spoon in a bowl of Jell-O,” says Christina Tringides, a postdoctoral fellow at the Harvard John A. Paulson School of Engineering and Applied Sciences and lead author of the December 2022 journal article. “When we breathe, our brain has a little bit of micromotion. If you are gently shaking the bowl of Jell-O, with the spoon in its center, over time there is going to be a small, spoon-induced cavity in the Jell-O. In terms of [a device], it means there’s tissue damage and scarring that occur over time.”
The soft-brain interface described in the December 2022 article consists of a web-like scaffold made of a hydrogel—a type of polymer material that can absorb large amounts of water without dissolving, like a very soft sponge—extracted from seaweed. Researchers added carbon nanotubes and graphene flakes as conductive materials, which enable the hydrogel to transmit electrical impulses to and from the brain. And they used an innovative freeze-drying process to give the hydrogel a porous structure, with tiny pockets that provide a microenvironment to support the growth of brain cells. The result is an extremely flexible, conductive—and partially living—BCI.
“Hydrogels have the same mechanical properties and even the same mechanical character, called viscoelasticity, as the brain,” Tringides says. This could help reduce problems with rejection. “With rigid or stiff devices, one of the first things that happens in any implant is that the body builds a fibrotic scar to contain the implant and wall it off from the rest of the body since it is seen as foreign. Hydrogel electrodes shouldn’t have that problem.”
In the lab, the researchers seeded the hydrogel scaffold with human neural progenitor cells—“blank slate” cells that can transform into a variety of specific cell types—and after pulsing them with electrical current, discovered that they had “formed networks of lattice-like structures on the scaffold and differentiated into multiple cell types with specific features.” Some of the important types of brain cells discovered to be growing in the hydrogel include astrocytes, which aid in neural repair, and oligodendrocytes, which create the myelin sheath that insulates long, skinny nerve fibers called axons. Growing myelinated axons has been an ongoing challenge in developing living models of the brain.
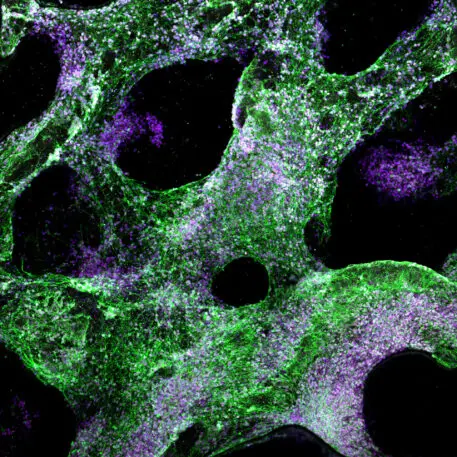
Tringides—who has filed for a patent on the technology along with Harvard colleague and study co-author David Mooney—believes that soft electrodes containing living cells would permit more natural cell-to-cell contact than metal versions, and better integration with the brain. “In the theoretical case of BCI, the device implanted would now really mimic the architecture and composition of the brain,” she says.
The new hydrogel is not yet FDA approved, so any device incorporating it is likely 5 to 10 years from being used in humans, says Tingrides. More immediately, she and her colleagues plan to use the hydrogel scaffolds to create laboratory models of the brain that could shed light on the cell-differentiation process and how, for example, neurons form connections with each other and other supporting cell types. Eventually, Tingrides hopes these brain models could replace animal testing: “We could apply a drug or [replicate a brain] injury to see how these networks of cells are affected and, then, how we could recover them. So many drugs [that go through] in vitro testing in plastic fail in animal testing. Because the hydrogel really matches the properties of the brain, the cells grow in a really similar way to how they would in the body. We think [this] is the better place to test and then transition these drugs.”
Recognize your brand’s excellence by applying to this year’s Brands That Matter Awards before the early-rate deadline, May 3.