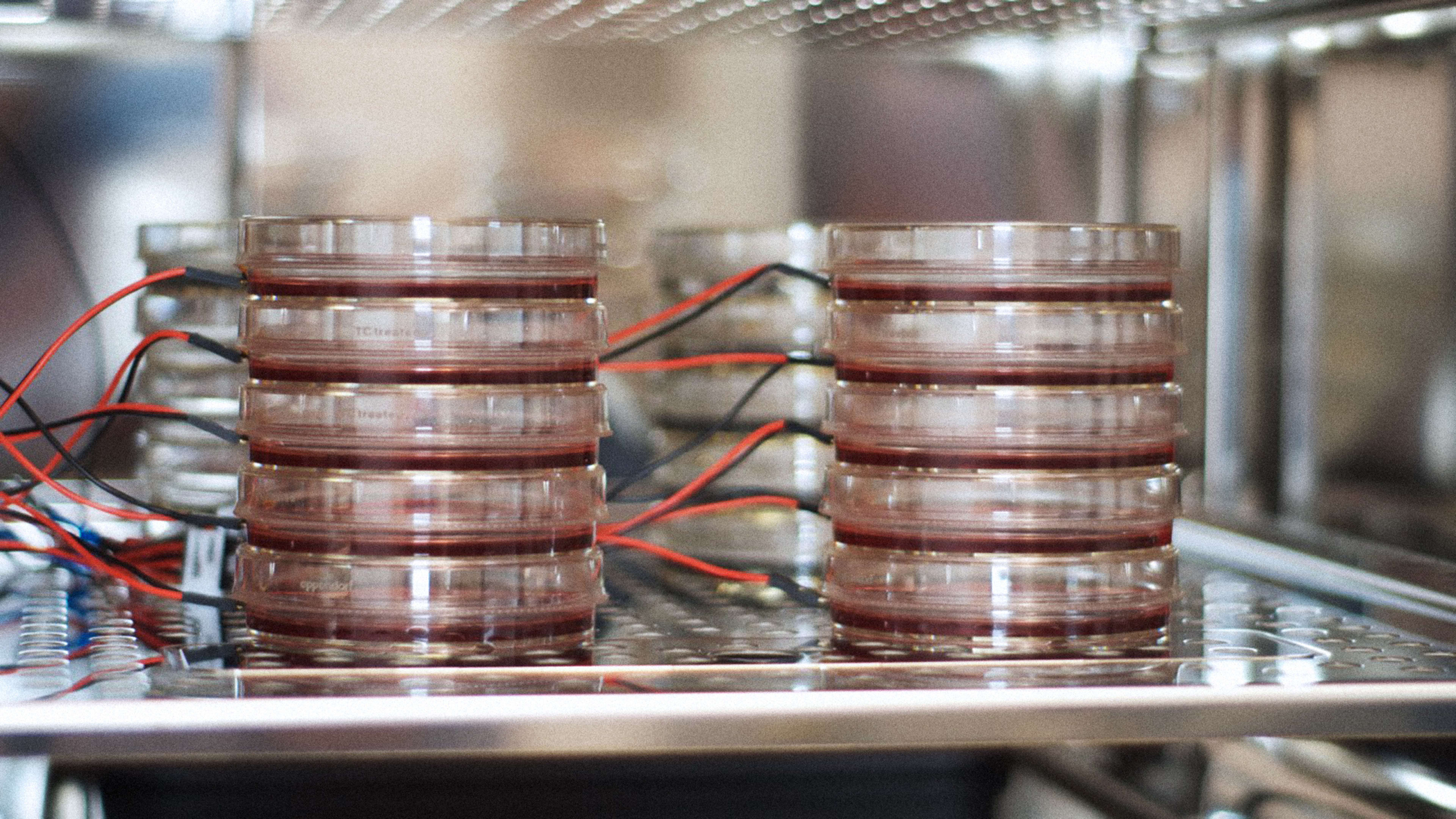
It looks like an elongated blob: a pulsating, concave blob, suspended on wires. It doesn’t seem like much when I first see it in the flesh–except that it may represent the future of drug testing.
I’m standing in the lab at Tara Biosystems, a startup near the East River, in New York City. CEO Misti Ushio is showing me around the newly opened space. As I look through a microscope, the blob, which is made of artificially created heart tissue, starts to jiggle in response to an electric current. It’s like a real heart reacting to stimulus from the brain: pumping and pumping. It’s just a lot smaller than a real human heart–about 3 millimeters long in its non-magnified version.

“It’s like a tissue machine,” says Ushio. “We have a pipeline at different stages of development, so we’re always ready if we need to do a study and we can react quickly.” The lab’s fridge contains about 200 tissue cultures at any one time, she says.
Tara currently does mostly toxicology testing with the platform–that is, making sure that the heart tissue isn’t harmed when it’s bathed in a drug solution. That offers an early-stage warning system for pharma companies. Instead of waiting for human trials at the end of the drug development process to reveal toxicology problems, they can now see if there could be problems before real humans are ever involved. More than 10 drug groups are already using Tara’s service, Ushio says (she won’t name names as yet).
“Over the summer we began to get to a stage where we didn’t really have to convince people why this is important. They know why it’s important. They just want us to demonstrate that we can do it.”

And toxicology is just the beginning. The next stage is to take diseased heart tissue and subject that to drug testing: for the tissue to actually be used in the same way real humans or animals are used in drug trials. The platform could even allow companies to test personalized types of heart tissue, showing how an individual’s heart tissue may react to a drug, not just a generic form of heart tissue.
“We’ve standardized on one cell line so we can show that our process is reproducible and we can get the same tissue results every single time,” she says. “The future is to look at the difference between your heart and my heart and try to elucidate which types of people would benefit from the new medicines and which ones would be at risk based on their genetic background or medical history.”
Ushio hopes the data being accumulated in Tara’s experiments will help the platform to become predictive. The data will inform algorithms that can forecast how types of heart tissue will react in the presence of many strains of drugs. She sees the possibility of reducing drug testing time frames from 10 years (as now) to perhaps as little as one year. A combination of stem cell science plus tissue engineering plus powerful computers would substitute for a lot of the trial-and-error work currently done by humans in labs, she says.
There’s also the possibility of doing without animal testing altogether for new drugs. Instead everything could be tested on artificially rendered versions of human organs before the very last stage of the process, when actual humans have to be brought into the process. And not just for heart cells, but for all the main organs of the body.
“If you had a human on a dish–a collection of all the important tissue types–you really may start to eliminate animal testing altogether. It’s not a today thing. But in the near term, this could help us use animals just for higher value projects, where you already have a lot of information [from other testing methods],” she says.
Staring at the human heart tissue blob in Tara’s lab, I had a somewhat queasy feeling about human organs being created out of thin air. But it also seemed like a major advance that we might be able to create useful, personalized drugs at a faster rate than now, and that we might not subject pigs and monkeys to any more unnecessary pain in doing so.
Recognize your brand’s excellence by applying to this year’s Brands That Matter Awards before the early-rate deadline, May 3.