AliveCor, the digital health company led by ex-Googler Vic Gundotra, says the Food and Drug Administration has approved its EKG band accessory for the Apple Watch. Gundotra says the Kardia Band, as the product is called, is the first Apple Watch health accessory to get FDA clearance.
Kardia Band attaches to the Apple Watch like any other replaceable watch band. The user rests a finger on the sensor pad embedded in the band, allowing an EKG reading to be taken. (Electrocardiograms detect irregularities in the electrical impulses that trigger the contractions in the four chambers of the heart.) The Kardia Band transmits its EKG reading to the Apple Watch (via a high-pitch audio signal) where it’s displayed in real time as a moving waveform. When the 30-second EKG is finished, the user can view it on their phone or easily send the results as a PDF to their physician.

Gundotra says the Kardia Band will be useful to younger people who have diagnosed heart conditions, and by people in their 40s or older who have congenital heart disease in their families.
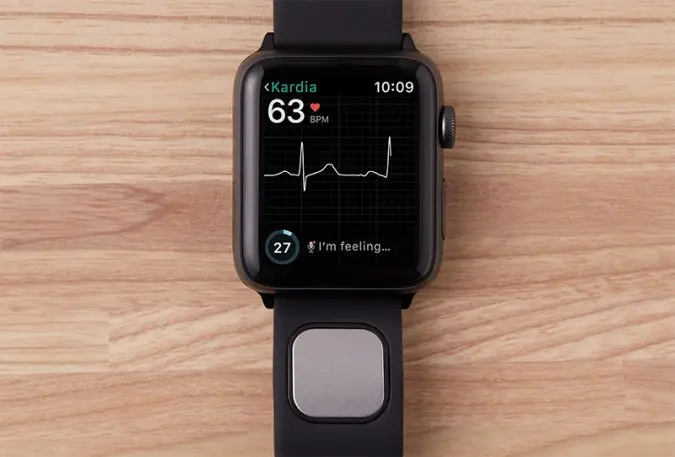
Then there’s the AI. The new version includes something called SmartRhythm, a set of machine learning algorithms that run on the Apple Watch and use real-time and historical activity and sensor data to predict an acceptable heart rate range for the user throughout the day. If the user’s heart rate goes outside that range a number of times or for long periods of time, the AliveCor app running on the watch suggests that the user take an EKG reading.
That prompting function is important to doctors taking care of heart patients at risk of atrial fibrillation, Scripps cardiologist Dr. Eric Topol told me.

“If you can direct them when to do the EKG it’s going to markedly increase the number of EKGs taken.” That gives the cardiologist more diagnostic data to work with, but it also feeds more training data to AliveCor’s AI, making it better and better about reading the clues in the heart rate data.
“I think we’ll learn much when [Kardia Band] gets out there in big numbers,” Topol said.
Gundotra said it took time for him and his company to learn how to work with the FDA. Seeking approval for a digital health product is an expensive and time-consuming process that often seems foreign and off-putting to Silicon Valley companies. Gundotra, an ex-Google executive who oversaw the development of Google Photos and Google+, says it took some time for he and his team to adapt to the agency’s methodical, evidence-based way of doing things.
It’s a worthy mission. Just look at the numbers. Cardiac disease kills more people each year than all types of cancer combined. The kicker is that people who have atrial fibrillation live with it for an average of 1.7 years before it is actually diagnosed, and the diagnosis often occurs after something terrible has happened, like a stroke. That’s where AliveCor could really help–an early warning could save lives.
Kardia Band is available at AliveCor.com and on Amazon starting today for $199. It requires subscription to AliveCor’s premium service for $99 a year.
Correction: An earlier version of this story said Vic Gundotra is the founder of AliveCor. Actually, the company was founded by Dr. David Albert. Gundotra is the CEO.
Recognize your brand’s excellence by applying to this year’s Brands That Matter Awards before the early-rate deadline, May 3.
